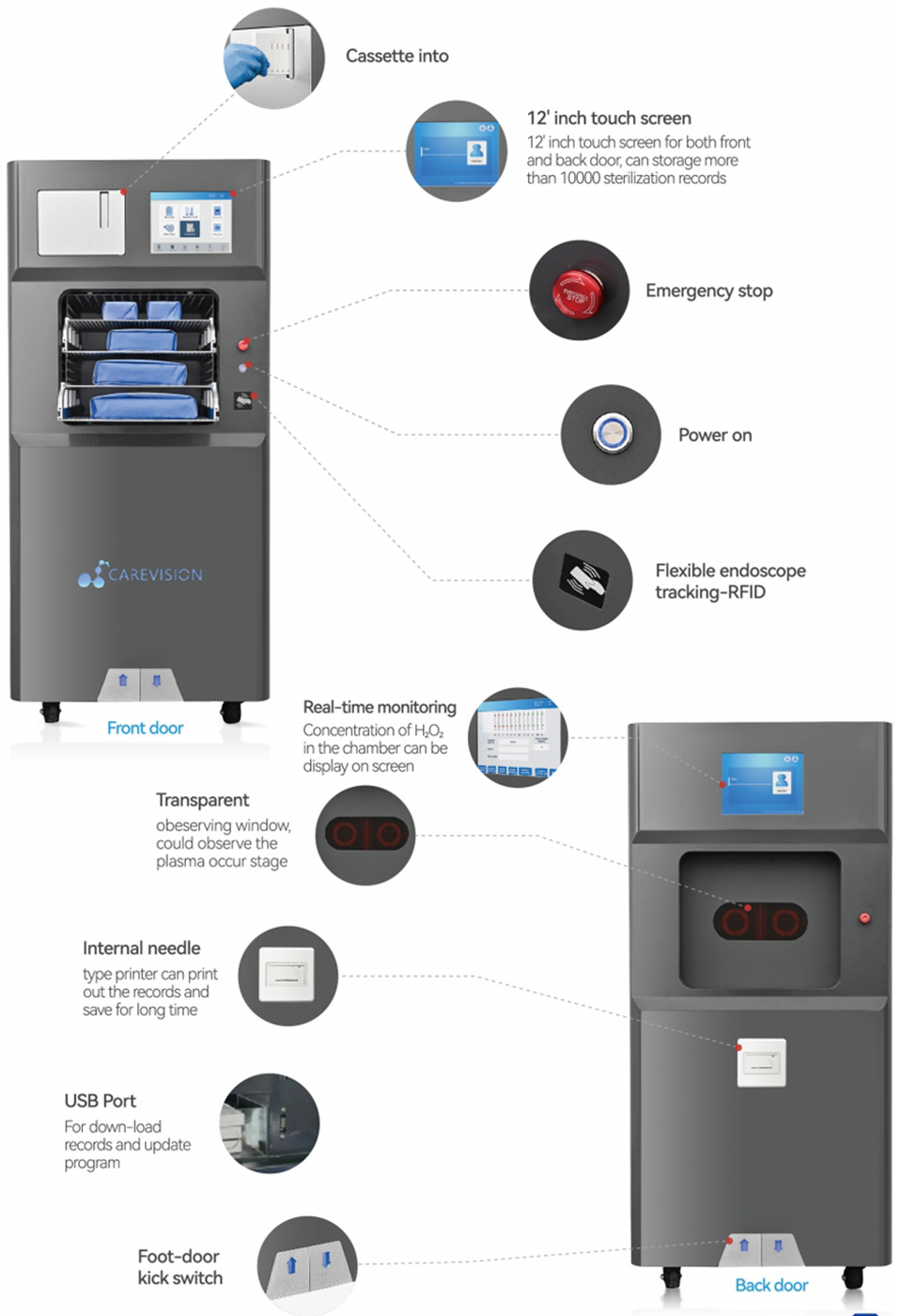
LOW TEMPERATURE VAPORIZED HYDROGEN PEROXIDE PLASMA STERILIZER CVM-120S/D,CVM-150S/D
- Commodity name: LOW TEMPERATURE VAPORIZED HYDROGEN PEROXIDE PLASMA STERILIZER CVM-120S/D,CVM-150S/D
Product Classification:
Key words:
Low-temp vaporized hydrogen peroxide plasma sterilization technology was patented in 1987 and launched in 1993 in USA.Plasma state is called as the 4th stage of matters by scientist following after the solid, liquid and gas in our universe.
Product Details
Sterilization principle:
Low-temp vaporized hydrogen peroxide plasma sterilization technology was patented in 1987 and launched in 1993 in USA.
Plasma state is called as the 4th stage of matters by scientist following after the solid, liquid and gas in our universe. The necessary condition to form plasma stage is by adapting the RF or microwave technology to generate into the charged particles which present as majority radical form in the extremely sealing vacuum container The radical is an atom with unpaired electrons, which can interact with enzymes and nucleic acid which is the essential cellular components to form into microorganism , in order to destroy the microorganism's metabolize, the high concentration of vaporized hydrogen peroxide after purification will diffusion into the chamber, at this time, combined with plasma to form a variety of free radicals like hydroxy and peroxyhydoxyls which can block the metabolize of microorganism, so as to achieve the purpose of sterilization. in the technology of low-temp vaporized hydrogen peroxide plasma sterilization, the plasma is not directly involved in the sterilization process, but can accelerate the decomposition of residue after sterilization process if apply the plasma technology into the vaporize hydrogen peroxide sterilization processing, which helps to reduce the exposure of harmful gases discharged after sterilization cycle and reduced the injury to hospitals nurses.
Main low-temp sterilization methods compare.
| Sterilant | Sterilization mechanism | Temp(℃) | Concentration |
| EO | Alkylation | 30-60 | 600-1200 mg/L |
| LTSF | Alkylation | 60-80 | 8-15 mg/L |
| VH₂ O₂ plasma | Oxidize | 40-50 | 6-10 mg/L |
| Peracetic acid | Oxidize | 50-55 | 0.20% |
Applicablerange
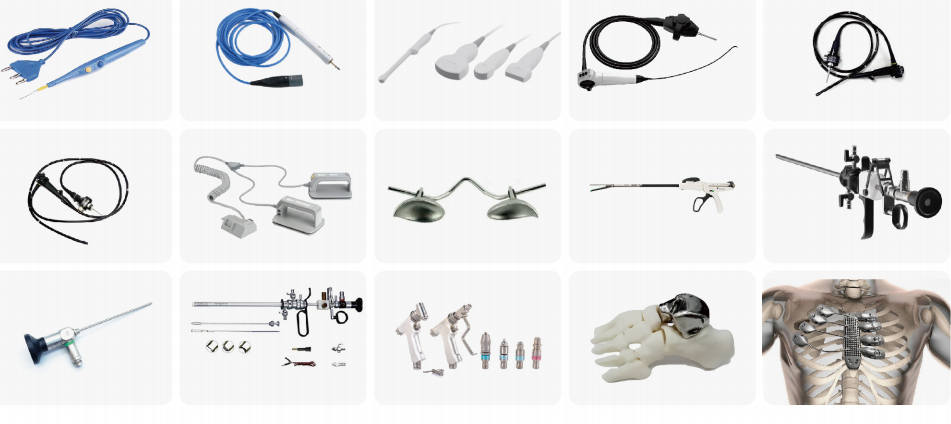
>95%medicalinstrumentsandmaterialsareapplicableforlow-tempvaporizedhydrogenperoxideplasmasterlizationtechnologyincludingmajorityinstrumentsandmaterialwhichareavalableforhightempmoisturesteamsterilization.Vaporizedhydrogenperoxideplasmasterilizationtechnologyisespeciallyusedfortheinstrumentsandmaterialwhichcannotapplicableforhigh-tempmoisturesteamsterilizersuchasteflonPTFElumens,stainlesssteellumens,electroniclumens,othermentalalloymaterials,includ.ingbutnotlimitedtothefollowinginstruments:
 、
、
Advantages

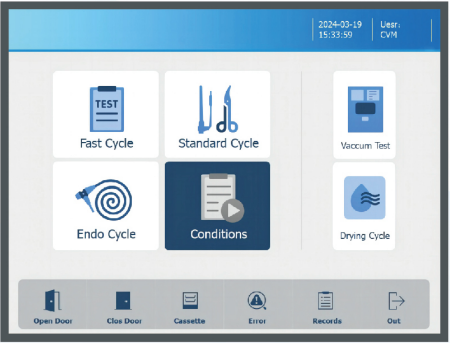
4 programs as standard configuration (e.g.CVM-120S-Total 150L)
| Test cycle (35mins) | Genera cycle(45mins) | Endo cycle(48mins) | Drying cycle(20mins) |
|
▶Flash surface sterilization for olidsurgical instruments or deviceswithout hollow lumens ▶Indicated engineer's maintenanceor service for the sterilizer |
▶Mix loading ▶Solid instruments ▶Solid instruments ▶Super long lumens ▶Other devices which not applicablefor steam sterilizer and couldcompatible with re-processindmedical instruments Note: this program cannot selectfor flexible endoscope's sterilization |
▶Flexible endoscope ▶Flexible endoscope's tracking Note: read the lFU of flexibleendoscope to define the correctsterilization method accordingly |
▶Vacuum+hot air drying Note: it is forced to initial underflexible endoscope program, andoptional under Test cycle and General cycle |
| 1 ampule | 2 ampules | 2 ampules | 无 |
 |
 |
 |
 |
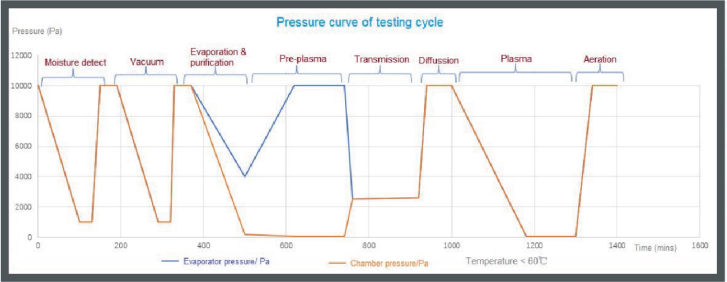
Sterilization effectiveness of half sterilization cycle
| Material | Type | Diameter | Length |
| Stainless steel lumen | Through out | 0.7mm | 600mm |
| PTFE (Teflon) lumen | Through out | 1mm | 4000mm |
Multiple chamber sizes and models to meet with differenthospitals’requirements
| Model | Usable chambervolume | Usable chambervolume | Suggestion |
| CVM-60S | 80L | 60L |
▶Veterinary, ophthalmology, dental clinic and laboratories ▶Surgery room of hospitals (reprocessing for emergency surgery,rapid turnover and short transit distance) ▶Limited room size of CSSD (single room) |
| CVM-100S | 133L | 108L |
▶urgery room of hospitals (reprocessing for emergency surgery,rapid turnover and short transit distance) ▶CSSD of hospitals (single room) |
| CWM-120S;CVM-120D | 150L | 120L |
▶Medium size CSSD of hospitals (single room) ▶Large size CSSD of hospital which need though out door project |
| CVM-150S:CVM-150D | 186L | 154L | |
| CVM-200S-DUAL100 | 266L | 200L |
▶Small footprint, limited room size for low-temp sterilization solution ▶Surgical instruments quantity increased CSSD ▶CSSD which need to increase the turnover rate of surgicalinstruments rapidly |


Accessories and consumables

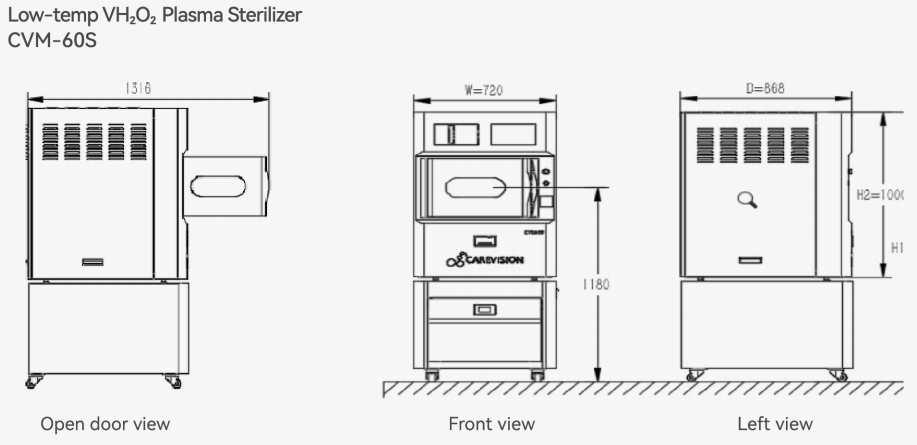
Installation dimensions (mm)

Specifications of CVM series Low-temp Vaporized H2O2 Plasma Sterilizer
| Image |  |
||
| Model | CVM-120S/D | CVM-150S/D | |
| Door type | Automatic sliding single/double door; safty protection | ||
| Installation method | Floor installation with movable caster | ||
| Chamber type | Single / through out chamber | ||
| Total chamber volume | 150L | 186L | |
| Usable chamber volume | 120L | 154L | |
| Chamber material | Aircraft level aluminium | Aircraft level aluminium | |
| Chamber shape | Rectangle | Rectangle | |
| Power supply | 3 Phases; 380V 50Hz | ||
| Programs (4 kinds) | Fast: 35mins | Fast: 35mins | |
| Standard: 45mins | Standard: 48mins | ||
| Flexi Endo: 48mins | Flexi Endo: 52mins | ||
| Vacuum Drying: 20mins | Vacuum Drying: 20mins | ||
| Software function | Humility detection | ||
| H2O2 concentration detection (optional) | |||
| Power display of plasma stage | |||
| Pressure display | |||
| Time display | |||
| Temp display | |||
| Input power | ≤4200W;150L≤4800W | ||
| Constent temp power | 1200W | ||
| Standby power | About 25W | ||
| Noise | ≤60dB | ||
| Operation environment temp | 5℃~40℃ | ||
| Operation environment humidity | 30%~95%(No frosting) | ||
| Operation atmospheric | 700hPa~1060hPa | ||
| Dosage volume of H2O2(ml) | Fast: 4.5mL/cycle; Standard: 9mL/cycle; Flexi Endo: 9ml/cycle | Fast: 5mL/cycle; Standard:10mL/cycle; Flexi Endo: 10ml/cycle | |
| Cycle QNT/cassette | Fast:12 cycles; Standard:6 cycles; Flexi Endo: 6cycles | Fast:12 cycles; Standard:6 cycles; Flexi Endo: 6cycles | |
| Chamber working temp | 45℃~60℃ | ||
| Maximum chamber vacuum degree | <80Pa | ||
| Machine dimension(W*D*H) | 800mm*1100mm*1790mm(Single door) 820mm*1130mm*1790mm (double door); | 820mm*1070mm*1790mm (single door); 820mm*1130mm*1790mm (double door); | |
| Installation space(W*D*H) | 1400mm*1500mm*2000mm(Single door) 1400mm*1600mm*2050mm (doubledoor); | 1400mm*1500mm*2050mm (single door); 1400mm*1600mm*2050mm (doubledoor); | |
| Instrument shelf | Type: movable | ||
| Qantity and load capcity: 2 (4 available) shelves; total 30KG, 15kg/shelf | |||
| N.W. | 460Kg | 500kg | |
| G.W. | 520Kg | 600kg | |
| Panel material | Q235 carbon steel with plastic spraying painting | ||
| Maintenance | 6 months | ||
| Guarantee period | 2 years | ||
| Touch screen | 12inch; TFT true color; touch screen | ||
| Printer | Internal needle printer | ||
| Data record and tracking | Check and print at any time | ||
| Internet Of Things ( IOT) | port opening with HIS/CSSD/CME/CSD system, USB port, RFID of endocope sterilization tracking | ||
online message